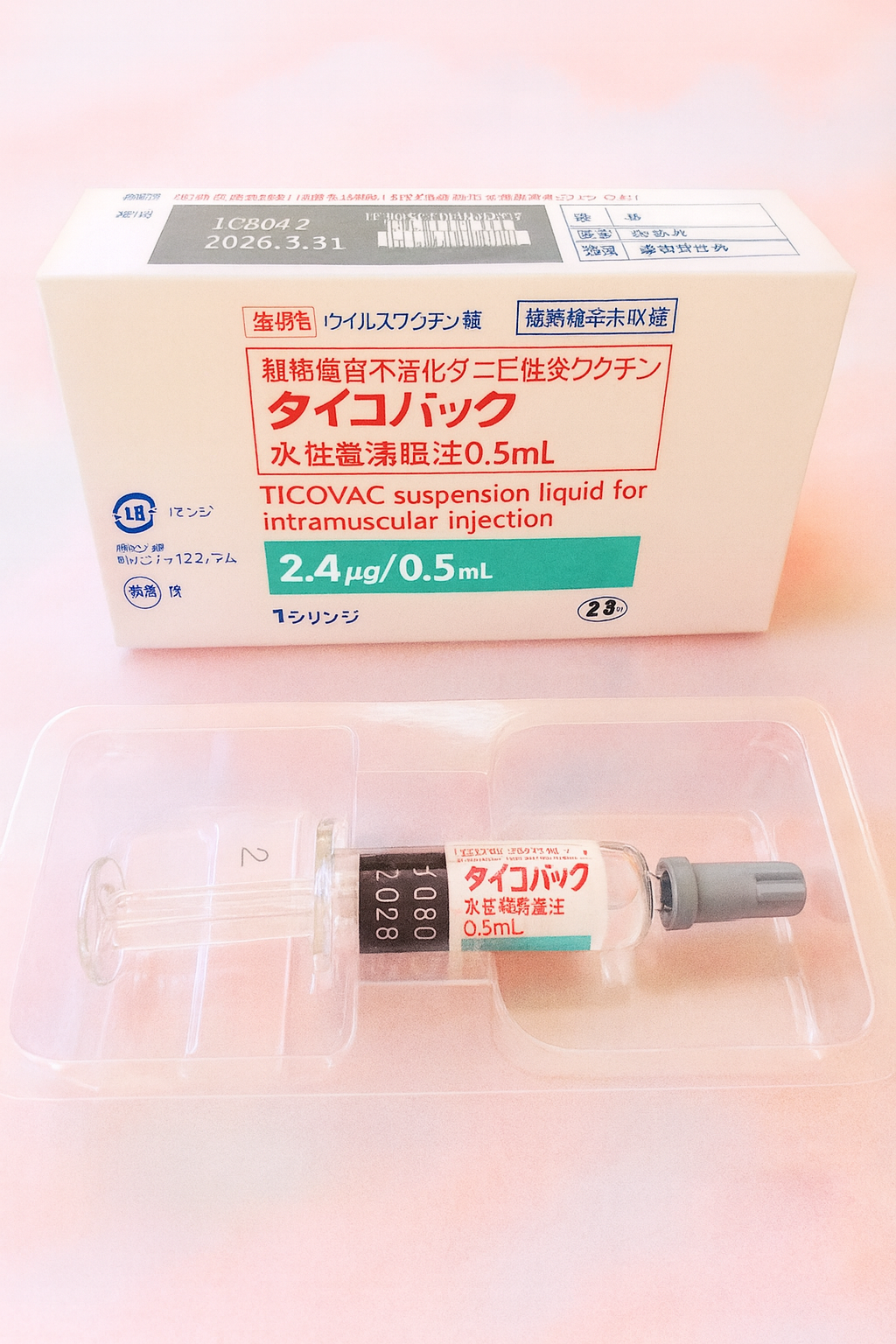
Pfizer Japan has launched TicoVac®, the country’s first approved vaccine to prevent Tick-Borne Encephalitis (TBE), available in adult (0.5mL) and pediatric (0.25mL) formulations. TBE is a serious viral infection transmitted by ticks, with symptoms ranging from fever and headache to severe neurological issues. The Far Eastern subtype, found in Hokkaido, has a fatality rate over 20%, with long-term complications in many survivors.
While previously rare in Japan, recent cases in Hokkaido and antibody findings in multiple prefectures suggest the virus may be more widespread. Limited awareness and testing may mean many cases go undiagnosed.
TicoVac® has been used safely in Europe since the 1970s and was officially approved in Japan in March 2024. It is recommended for residents in at-risk regions and travelers to endemic areas. Contact us to learn more or schedule your vaccination.
*Three doses and 5-12 months will be needed to complete the initial series.